 Research led by Dr Steve Liddle has resulted in publication of the first uranium-nitrogen triple bond, which has eluded scientists for decades, in the leading journal Science. Molecules which contain multiple bonds between a metal and elements such as carbon, nitrogen, and oxygen have attracted intense interest over many years. This is due to interest in their bonding, and the fact that such molecules find major applications in synthesis as exemplified by the 2005 Nobel Prize for Olefin Metathesis which involves metal-carbon multiple bonds.
Research led by Dr Steve Liddle has resulted in publication of the first uranium-nitrogen triple bond, which has eluded scientists for decades, in the leading journal Science. Molecules which contain multiple bonds between a metal and elements such as carbon, nitrogen, and oxygen have attracted intense interest over many years. This is due to interest in their bonding, and the fact that such molecules find major applications in synthesis as exemplified by the 2005 Nobel Prize for Olefin Metathesis which involves metal-carbon multiple bonds.
For uranium, double bonds to carbon, nitrogen, and oxygen are well known. However, attempts over several decades to prepare molecular uranium-nitrogen triple bonds (known as uranium-nitride) had always resulted in bridging nitrogen centres or species that were too reactive to isolate. Previous attempts to prepare uranium-nitrogen triple bonds have required temperatures as low as 5 Kelvin (-268 °C) — roughly the equivalent temperature of interstellar space — and have therefore been difficult to work with and manipulate, requiring specialist equipment and techniques.
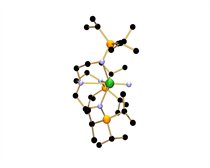
The uranium-nitride was accessed by using a very ‘bulky’ nitrogen ligand (an organic molecule bonded to a metal) to wrap around the uranium centre and to create a protective pocket in which the nitride nitrogen can sit. The nitride was stabilised during the synthesis by the presence of a weakly bound sodium cation which blocked the nitride from reacting with any other elements. In the final stage, the sodium was gently teased away, removing it from the structure and leaving the final, stable uranium-nitride triple bond.
Dr Liddle said: “The beauty of this work is its simplicity – by encapsulating the uranium-nitride with a very bulky supporting ligand, stabilising the nitride during synthesis with sodium, and then sequestering the sodium under mild conditions we were able to at long last isolate this previously elusive linkage.” Dr Liddle added: “A major motivation for doing this work was to help us to understand the nature and extent of the covalency in the chemical bonding of uranium. This is fundamentally interesting and important because it could help in work to extract and separate the 2 to 3% of the highly radioactive material in nuclear waste.”
The breakthrough could have future implications for the nuclear energy industry - uranium-nitride materials may potentially offer a viable alternative to the current mixed oxide nuclear fuels used in reactors. However, uranium-nitride materials are usually prepared by high temperature and pressure routes which gives impurities that cannot be removed. The successful synthesis of a uranium-nitride could allow a milder method of preparing uranium-nitride materials.
The research was supported by the UK National Electron Paramagnetic Resonance (EPR) Facility, funded by the Engineering and Physical Sciences Research Council and based in the Photon Science Institute at The University of Manchester. The uranium-nitride contains an unpaired electron and by using EPR spectroscopy it was possible to interrogate the nitride and it was found that it behaves differently from similar compounds prepared at Nottingham.
Professor Eric McInnes, from the EPSRC National EPR Facility at The University of Manchester, said: “EPR spectroscopy can give detailed information about the local environment of unpaired electrons, and this can be used to understand the electronic structure of the uranium ion in this new nitride. It turns out that the new nitride behaves differently from some otherwise analogous materials, and this might have important implications in actinide chemistry which is of vital technological and environmental importance in the nuclear fuel cycle.”
The research has been funded and supported by the Royal Society, European Research Council, the EPSRC, and the UK National Nuclear Laboratory.
Citation: D. M. King, F. Tuna, E. J. L. McInnes, J. McMaster, W. Lewis, A. J. Blake, S. T. Liddle, Science, 2012, 337, 717-720. Figure key: uranium=green; silicon=orange; nitrogen=blue; carbon=black; hydrogen atoms omitted for clarity.
Posted on Friday 29th June 2012