Glycoprotein Ib-IX-V complex
The GPIb-IX-V complex is an abundant membrane receptor complex originating in megakaryocytes and occurs on the surface of platelets (Li and Emsley, 2013). It primarily functions to mediate the first critical step in platelet adhesion, by binding to von Willebrand factor (vWF) on damaged sub-endothelium under conditions of high fluid shear stress (McEwan et al., 2009, López et al., 1998). Although the primary ligand for the GPIb-V-IX receptor is vWF, it can also bind to a number of other ligands such as thrombin, P-selectin, factor XI, factor XII, high molecular weight kininogen as well as bacteria. GPIb-IX-V has been implicated in thrombotic disease processes such as stroke or myocardial infarction (McEwan et al., 2009, Li and Emsley, 2013).
Molecular Structure: An overview
GPIb-IX-V consists of four subunits : GPIbα (MW 135 kDa), GPIbβ (MW 26 kDa), GPIX (MW 20 kDa) and GPV (MW 82kDa). The complex is assembled such that GPIbα, GPIbβ and GPIX form a highly integrated protein complex in a 1:2:1 stoichiometry; and this associates weakly with GPV resulting in an overall stoichiometric ratio of 1:2:1:1 (Li and Emsley, 2013, Du et al., 1987, Luo et al., 2007, Mo et al., 2012)
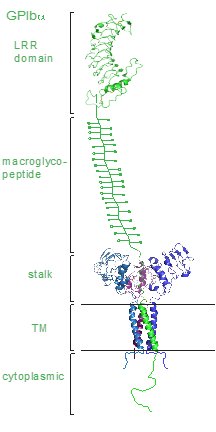
Each subunit of the complex is a type I transmembrane (TM) protein which consists of a leucine-rich repeat (LRR) ectodomain (extracellular domain), a single transmembrane helix, and a relatively short cytoplasmic tail (Li and Emsley, 2013, McEwan et al., 2011).
The quaternary organisation of the receptor is facilitated by covalent and non-covalent interactions. The GPIbα subunit is linked to two GPIbβ subunits via membrane-proximal disulfide bonds (Figure 2), while GPIX associates itself tightly through non-covalent interactions with GPIb (Du et al., 1987, Luo et al., 2007, McEwan et al., 2011). The concomitant expression of all three subunits is required to allow the effective expression of GPIb-IX on the platelet cell surface and analysis of receptor expression in transfected Chinese hamster ovary (CHO) cells has further supported that the interaction between these subunits also acts to stabilize them (Li and Emsley, 2013).
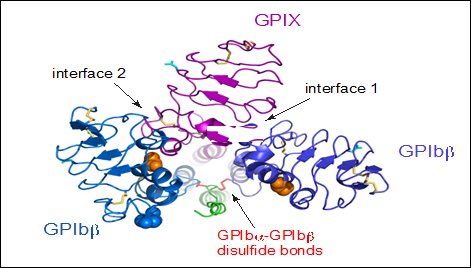
View from the top of the membrane proximal portion of GPIb-IX showing the disulfide bonds between GPIbα and GPIbβ (Li and Emsley, 2013).
Each of the four subunits (GPIbα, GPIbβ, GPIX and GPV) is part of the leucine rich repeat motif superfamily. These leucine rich repeat sequences average 24 amino acids in length either occurring singly or in tandem flanked by conserved N-terminal and C-terminal disulfide loop structures (López et al., 1998).
Human GPIbα is the product of a gene on chromosome 17 specifically 17p12, GPIbβ is the product of a gene on chromosome 22 specifically 22q11.2, while GPV and GPIX are products of genes found on chromosome 3 specifically 3q21 and 3q29 respectively (Lanza, 2006). Under normal conditions, all four molecules are expressed exclusively in the platelet lineage. GPIbα, GPIbβ and GPIX are necessary for the effective biosynthesis of the receptor and are closely associated at the platelet membrane. Typically, a lack of a single subunit significantly decreases the surface expression of the entire receptor complex (Nurden, 2005, Lanza, 2006)
GPIbα
GPIbα consisting of 610 amino acids is the major subunit and contains all known extracellular ligand-binding sites of the complex of which two are shown in Figure 3: The A1 domain of von Willebrand factor (vWF) has a binding region as marked in the N-terminal domain of GPIbα; while the thrombin binding site is contained in a conformationally flexible acidic residue-rich sequence containing sulfated tyrosines (Emsley and Li, 2013, López et al., 1998).
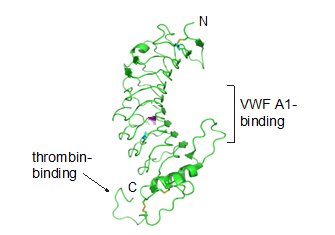
Ribbon diagram depicting the crystal structure of the GPIbα N-terminal domain including the vWF A1 and thrombin binding sites (Li and Emsley, 2013)
We have previously determined the crystal structure of the GPIbα N-terminal leucine rich repeat domain. This shows the structure of a single disulfide bond between Cys4 and Cys17 in the N-capping region, and two disulfide bonds (Cys209-Cys248 and Cys211-Cys264) in the C-capping region. Furthermore, there are seven tandem leucine rich repeats and their flanking sequences in the central parallel β-coil region. This parallel β-coil region is made up of three sided coils stacked in layers and contains two asparagine residues (Asn21 and Asn159), which serve as N-glycosylation sites. Following the leucine rich repeat domain is the acidic residue-rich sequence containing sulfated tyrosines, the highly O-glycosylated macroglycopeptide, a stalk region of about 40 to 50 residues, a single transmembrane sequence and finally a cytoplasmic tail containing 96 amino acid residues which includes residues such as Ser587, Ser590 and Ser609 that can be phosphorylated (Li and Emsley, 2013, López et al., 1998)
GPIbβ, GPIX, GPV
GPIbβ contains 181 amino acids. In the extracellular domain (ectodomain), both the N-capping and C-capping regions, which flank the leucine rich repeat sequence, contain two interlocking disulfide bonds. Furthermore, there is only a single leucine-rich repeat giving rise to a much less curved parallel β-coil region as compared to that in GPIbα. GPIbβ contains only one N-glycosylation site (Asn41) and is disulfide linked to GPIbα immediately proximal to the plasma membrane of the platelet via Cys122 located at the junction of the extracellular and transmembrane domains (López et al., 1998, Li and Emsley, 2013).
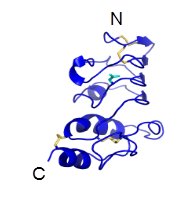
Ribbon diagram depicting the crystal structure of the GPIbβ extracellular domain (Li and Emsley, 2013).
GPIbβ is 180 amino acid long. The GPIbβ cytoplasmic domain has a sequence of 34 amino acids. The region adjacent to the membrane is enriched in basic residues and Ser166 found more distally is phosphorylated and may have a role in platelet cytoskeletal rearrangement (Li and Emsley, 2013, López et al., 1998).
GPIX contains 160 amino acids. The extracellular domain, which also only has a single leucine rich repeat sequence shares more than 45% sequence identity with homologous GPIbβ. The GPIX cytoplasmic tail is short consisting of 8 residues and is not known to associate with intracellular proteins. (López et al., 1998, Li and Emsley, 2013).
The extracellular domain of GPV has 13 leucine rich repeats. This is followed by a stalk region, the transmembrane sequence and a short cytoplasmic tail rich in basic residues (Li and Emsley, 2013, López et al., 1998).
The GPV subunit is only weakly associated with the GPIb-IX part of the receptor complex through interactions between the transmembrane domains and has little impact on the surface expression of GPIb-IX, although GPIb-IX is required for efficient expression of GPV (Mo et al., 2012, Li and Emsley, 2013). Furthermore, GPV doesn’t appear to be critical for vWF binding or signal transduction (McEwan et al., 2011).
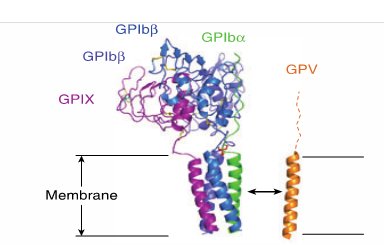
Interaction of GPV with GPIb-IX via transmembrane (TM) domains (Li and Emsley, 2013)