By Doug Wiles
The properties of
supercritical fluids
THE DEVELOPMENT OF INDUSTRIAL APPLICATIONS
Supercritical
Fluid Extraction (SFE)
Supercritical
fluids as reaction media
Supercritical fluids as reactant solvents
Supercritical Fluids
and the Environment
Potential
Alternatives to Supercritical Fluids
INTRODUCTION (top)
Traditionally liquids and gases are
assumed to be distinct and different phases, exhibiting different properties,
such as density and diffusivity. However it has been found that increasing the
temperature and pressure of a fluid beyond a certain value (depending on the
fluid), it becomes harder to describe the properties as simply liquid or
gaseous. This point is called the Critical Point. The bizarre fluids that occur
beyond this point have a combination of properties both liquid and gaseous. As
such, a variety of industrial and chemical uses have been found for these
fluids, which exploit the unusual combination of properties. The fluids are
given the name Supercritical fluids.
Supercritical fluids have low viscosities
and are highly compressible, like gases, and are relatively dense and can
dissolve a wide range of solid compounds, like liquids.
This essay will first attempt to describe
how these properties lead to the widespread uses of supercritical fluids,
primarily as solvents, in processes such as extraction, chromatography and
reaction chemistry. Secondly I will describe in a Chemical Engineering context
how supercritical fluid reactors are being developed and how the 'scale up' or
'scale out' considerations affect the industrial outcome of SCF (supercritical
fluid) research. I will include details of the environmental factors that are
driving the research into supercritical technology.
THE HISTORY (top)
The development in supercritical fluid
technology has been slower than would have been expected from the early date
that supercritical phase were discovered. This can be explained by the
difficulty of experimenting at high pressures and at high temperatures. The
early discovery was around the beginning of the Nineteenth century, by Cagniard
de la Tour. He was the first to notice that the gas/liquid phase boundary
disappeared when a certain temperature was exceeded. Supercritical Carbon
Dioxide was one of the first substances looked into by Andrews in 1869. However
the interesting solubility power that the fluids exhibited was demonstrated by
Hannay and Hogarth in 1880 [Hannay J.B. and Hogarth J., Proc. Roy. Soc.
(London) ser. A 1879 29 324]. They noticed that the solubility of Cobalt
Chloride in supercritical ethanol was greater than expected by extrapolating
subcritical data. Van der Waals then went on to look into the Thermodynamics of
supercritical fluids. Even though the natural physical effects of supercritical
fluids, as described above, were known by early researchers, it is only in the last 30 years that a rapid
development in supercritical knowledge has been achieved. The early patents
that some American engineers suggested were mostly ignored. However by the time
the end of the fifties arrived, a Russian, Zhuze [Zhuze T.P., Vesnik Akad.
NaukS.S.S.R 1959 29 47]. described a procedure for fractionating crude oil,
extracting earth waxes and obtaining Lanolin from wool fat with the aid of a
dense (supercritical gas). It was not however enough to spur great enthusiasm
into research into this area. This was achieved by researchers at the
Max-Planck Institute for coal research, who were looking into supercritical
extraction. Their development in the area resulted in other researchers
gathering enough information for there to be the first 'Symposium' in 1978 inn
Essen, with the topic: 'Extraction with Supercritical gases'.
THE CRITICAL
POINT (top)
The Critical point is the temperature and
pressure at which the liquid and vapour phases are no longer distinguishable.
It is best visualised on a phase diagram.
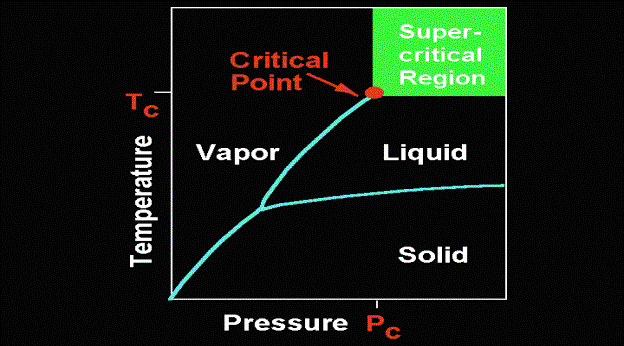
The lines that separate the liquid and
vapour and solid phases are phase boundaries. Crossing a phase boundary causes
a change in phase. They all cross at the Triple point. The liquid/vapour phase
boundary comes to a stop at a particular temperature and pressure. This is the
critical point. Beyond this is the critical region. Taking a vapour whose
temperature is beyond the critical point but whose pressure is not, and
increasing the pressure isothermally, we find we end up with a supercritical
fluid. Doing the same with a liquid whose pressure is beyond the critical point
and increasing the temperature isobarically, a supercritical fluid is achieved.
However in both cases a phase boundary was not crossed. Therefore, since
supercritical fluids can be accessed from both routes, it can be seen that the properties of a SCF
can only be described as a combination of liquid and vapour properties.
Beyond the critical point there is no
phase boundary, however it is possible to see what happens in this region if we
'extrapolate' the liquid/vapour phase boundary [J.A.Banister]. Increasing the
pressure, approaching the extrapolated phase boundary, causes the density to
increase rapidly. Therefore before the extrapolated phase boundary, the fluid
behaves more like a dense gas, and after the extrapolated boundary, more like a
dense diffuse liquid. Therefore by varying the pressure the density can be
changed.
The 'phase' that exists beyond the
critical point can therefore be 'accessed' from both the liquid region and the
gaseous region. Therefore if a reaction is taking place between two substances
in a different phase (liquid and gas), which is called a heterogeneous
reaction, the use of supercritical properties implies that the reaction can be
made to take place in the same phase, i.e. a homogeneous reaction. This is
called phase homogenisation. A homogenous reaction is often faster because
there are fewer mass transfer resistances. Homogenisation can also ease product
separation.
The critical point can be measured by
several methods.[www.nottingham.ac.uk/supercritical/scacoust.htm]. One of these is the Acoustic method, which
can be used to locate the critical point of reaction mixtures. The general
principle is that the speed of sound reaches a minimum at the critical point.
Therefore if a source of sound whose speed within the reaction mixture can be
measured is used and recorded against changes in temperature and pressure.
THE PROPERTIES OF
SUPERCRITICAL FLUIDS (top)
Certain 'Thermophysical' properties exist
which can be manipulated to selectively direct the progress of chemical
reactions. [Sundaresh Ramayya et al].
Variable Solubility
M. Jobling reported in her 1992 Thesis
(Organometallic Photochemical Reactions in Supercritical Fluids) that Hannay
and Hogarth first observed the pressure dependence of solubility of inorganic
salts in ethanol. They observed that increasing the pressure on a fluid caused
more salts to dissolve, and decreasing the pressure on a fluid caused more
salts to precipitate. They noted that beyond the critical point, there was a
dramatic increase in the amount of solute that would dissolve in the fluid.
Only a small increase of pressure was needed to increase the solubility after
the critical point was reached. Conversely decreasing the pressure by
increasing the volume of what is essentially a gas with dissolved constituents,
and the dissolved substances precipitate.
This phenomenon can be explained quite simply by imagining the increase
in mobility of the particles in the fluid as it becomes supercritical. The
greater the mobility the larger the ability to attract and hold ions within the
structure of the fluid. Also at supercritical pressures fluids tend to retain
their ionic properties when raised to higher temperature, for example, water
retains its ionic properties (high dielectric constant and ion product) to temperatures of 400C or more when the
density is raised to 0.4 gcm-3. [Sundaresh Ramayya et al].
This ability to change the solubility and
the density of a substance simply by changing the pressure is very useful and
can be applied to many separation techniques such as chromatography and liquid
extraction, as I will describe below.
Ion Product
For chemical Reactions involving polar
These properties cause some well known
physical effects in the environment. The solvent power of supercritical gases
is involved in geological processes through the influence of water on rock
formation [Ingerson E., Econ. Geol. 1934 29 454]. In this reference Ingerson
noted that solutions above their critical temperature may effect a considerable
transfer of material. He described how both volatile and non-volatile compounds
are transported under high temperatures and pressures. The volatile compounds
(having a lower critical point) often 'dissolve' the less volatile compounds (higher
critical points) and transport them to a point where the pressure on the
mixture is released and the compounds can be deposited. For example SiF4 (Tc
-1.5C), SiCl4(Tc230C), SnCl4(319) and TiCl4(358C) are all compounds with a low
critical point. These have been found to transport the less volatile compounds
found in magma, e.g. (FeCl2 (Tc 1450C), AgCl (Tc 2460C), Cu2Cl2
(Tc 2185C) etc. The transport occur when a confined gaseous solution, which is
under considerable pressure from overlying rock, moves through the pore space
and channels in the rock so slowly that there is no appreciable pressure relief
as pressure due motion away from its source. Water is the most important
solvent from the geolgic point of view,. Water above its critical temperature can
carry at least 5% of its weight of alkali halides and appreciable amounts of
silica [George C. Kennedy Econ. Geo. 1950 45 pg 629]. This reference details
the possibility of silica dissolving in water under high pressures and
temperatures. This effect also has a bearing on methane in petroleum formation
and migration [ref.3]. Knopf [Knopf A.
U.S. Geol. Surv. Prof. Paper 114 pp44-45 1918] wrote a paper describing the ore
deposits of the Yerington District, Nevada, saying that aqueous solutions in
the supercritical state may have been responsible for the formation of the
deposits.
The high solvent ability and tuneable
density of supercritical fluids such as carbon dioxide and water means that
they can be used as industrial solvents and reaction media that are non-toxic.
Non-toxic solvents are preferred because toxic solvents need substantial
investment in safe disposal mechanisms. Toxic solvents can get into the body
where they can affect the nervous system, the respiratory system, liver and the
skin (particularly after prolonged and repeated contact the solvents will
dissolve the protective skin lipids).
They may get in the body by inhalation of a volatile solvent. Another
possibility is for the solvent to pass through the skin and into the blood
stream. When the solvent is in the body
the solvent undergoes a metabolic change. Toxic solvents include
trichloroethylene, perchlorethylene and trifluroethane. Obviously water and
carbon dioxide will not cause these effects to the human body and are much
cheaper to produce, and are therefore much preferred.
TYPES OF
SUPERCRITICAL FLUID (top)
D.F. Williams notes that Carbon Dioxide is
a commonly used supercritical fluid. Supercritical Carbon dioxide is often used
as a replacement for organic solvents. It has many properties that make it a
good choice: it is very non-reactive; non-toxic; non-flammable; cheap (since it
is available in the atmosphere) and therefore environmentally friendly. Carbon dioxide has therefore become increasingly
considered as the man alternative to the problems of recycling or disposal of
organic solvents in industry. The non-toxic nature makes it ideal for use in
the food-industry, where contamination of the food produced by the organic
solvents used can be a problem. The cost of the carbon dioxide is not, however
the only expense to be considered since operating the system at high pressure
is always expensive. Operating at supercritical conditions increases the
extraction rate by 2.5 times compared to using liquid carbon dioxide [D.F.
Williams].
An example of a synthesis reaction that
can be carried out in supercritical carbon dioxide, is the Friedel Crafts
Alkylation reactions (Addition of an alkyl group to a benzene ring). These
traditionally require long reaction times and low temperatures, using dirty
catalysts, for example AlCl3 or H2SO4. Using supercritical CO2 allows reaction
conditions to be tuned to get high product selectivity. Solvent removal is also
easy using supercritical CO2.
[www.nottingham.ac.uk/supercritical/scwater.html].
In order to improve the solubility of
carbon dioxide it is sometimes modified to make it more polar. This can be done
with small amounts of ethanol.
Another example is demonstrated by
researchers at the Technical Research Centre, Finland, who are researching into
the extraction of organic pollutants from soil using supercritical CO2 as a
solvent. This is a simple process in which the scCO2 is pumped through the
soil, which is kept in a pressure vessel. The pollutants are recovered by
pressure reduction. (process 100 to 200 bar, temp, 30-70C). Chemical
Engineering Progress July 1995 - pg. 47 reports that supercritical fluids can
also be used to regenerate carbon. The scCO2 is used in place of the desorbing
solvent, extracting the adsorbate from the surface of the Carbon. The solvent
is recovered by reducing the pressure so that it is sub-critical. The
adsorbates being insoluble in the sub-critical fluid are separated as a liquid
from the sub-critical vapour. This is still not a widely used method due to the
cost of high-pressure equipment.
Water is also a widely used supercritical
fluid. It is a common solvent in everyday chemistry, but supercritical water is
a much better solvent. It has the effect of becoming a reaction causing
solvent, and since great control over the solubility is achieved by altering
the pressure reactions can be controlled as never before. Therefore
supercritical water is applied to areas of chemistry such as organic substance
reactions, where many steps are involved. The critical point of water is 374C
and 218 atm. There is an additional change in water when it reaches its
critical point, it turns from the traditional polar liquid to a non-polar one.
The acidity also increases in line with the other changes that I have already
detailed above. This happens because the dissociation of ions within the water
increases dramatically up to and after the critical point. A term often used to
describe the amount of dissociation is the ion product. The ion product of
water is as follows: Kw=[H+][OH-]. The concentration under the conditions of
room temperature and pressure will be 1*10(-14). As the pressure and
temperature is increased the value of the ion product increases rapidly.
Conditions of 34.5Mpa and 300C the value is 1*10(-11). This is an increase in
the hydrogen ion concentration by about 30 times that of RTP. Thus
supercritical water can be used as an 'acid catalyst'. This leads to scientists
researching into ways that supercritical water can be used in the total
oxidation of toxic organics in supercritical water.
THE DEVELOPMENT OF INDUSTRIAL
APPLICATIONS
The uses of supercritical fluids are
increasing rapidly. Much research is being done into new areas and into making
existing supercritical fluid technology more affordable. Below I will detail some of the technologies
that use supercritical fluids. The concept was first noted by Hannay and
Hogarth (ref. 2).
Supercritical
Fluid Extraction (SFE) (top)
Supercritical gas extraction is a
technique that makes use of the solvent power of supercritical fluids at
temperatures and pressures near the critical point. [D.F. Williams (ref.1)]. It
requires high pressures, and therefore densities for the solvent to have a
sufficient capacity. The advantage of solvent extraction with supercritical
fluids, according to Micheal E. Mackay et al [Michael E. Mackay and Michael E.
Paulaitis Industrial and Engineering Chemistry Vol.18 No.2 1979 pp149] is that
the enhanced solubility effect is completely reversible and very sensitive to
changes in temperature and pressure. Consequently, the separation process can
be controlled closely and is capable of highly selective.
D.F. Williams [Chemical Engineering
Science pp1769 1981] says the main advantage over distillation is that it can
take place at moderate temperatures, and therefore it is suitable for use in
the recovery of heat-labile substances such as foods and petroleum products.
D.F. Williams goes onto describe the various uses of supercritical fluids. He
says that their uses include the breaking of azeotropes and the selective
extraction of stright chain paraffins or of aromatics from other hydrocarbons.
He goes onto list the following:
* ScCO2 and scPropane are used in the
extraction of spices and flavours. The flavour and colour of such extracts tend
to be much better than for other techniques.
* Carbon dioxide has advantages over other
gaseous or liquid solvents, because it introduces no health hazards.
* Decaffeination of coffee. [D.F.
Williams] This went into commercial production in Germany in 1978. The coffee
beans having previously been soaked in water, are palced in a pressure vessel
and are extracted with carbon dioxide at 16-22 Mpa, 363K. The carbon dioxide is
continuously recycled. The caffeine diffuses out of the beans into the
supercritical phase, in which it is carried into a washing tower where it is
washed with water at 343-363K. After 10 hrs, all the caffeine has been
transferred from the beans to the wsh water, which is then degassed and the
caffeine is recovered by distillation. The caffeine is reduced from around 3%
to about 0.02%. It is found that the caffeine is selectively removed from the
coffee, no substances contributing to the aroma are removed.
* Nutraceuticals (such as ginger) and
vegetable oil are also extracted by this technique.
* Organics can be removed from wastewater,
for example NG and DNT.
* The production of void free particles
can be achieved using SFE to remove the gas from the powder.
* Carbon dioxide has been used to extract
anti-cancer drugs such as taxol, vincristine sulfate, AIDS drug such as
Michellamine B, and other compounds. Polymers are separated in the
pharmaceutical industry. [sc-times.com:applications].
[Chemical Engineering Progress October
1995 pg.36.] Fluid extraction as a process is used in different circumstances
to distillation. It has the advantage that moderate temperatures can be used,
so it can be used with thermally sensitive materials. It can also be used where
low relative volatilities and azeotropes are involved and where energy savings
are possible. It is however a slow process, requiring long reaction times.
Supercritical extraction can be used because of the solvent abilities and
variable properties of the supercritical fluid. SFE uses a fluid such as scCO2
in place of a liquid solvent. In contrasts to liquid/liquid extraction, which
runs at atmospheric pressure SFE is normally run around 1000-2000 psig. This
process is well beyond the research stage and is used commercially for the
extraction of caffeine from coffee and tea, and for the extraction of hops.
Supercritical pentane to recover liquid fuels from heavy oils, in a process
called Residue Oil Supercritical Extraction (ROSE). SFE's main advantages lie
in the non-toxic nature of the supercritical solvents used. This means that it
can find applications where there are concerns about the environment or worker
over-exposure. However the big problem is the cost associated with
high-pressure equipment.
Jobling noted in her thesis that SFE has
disadvantages, namely:
* Once the extraction is complete the
solvent must then be removed.
* The choice of supercritical fluid is
restricted to those substances that have a low critical point.
* SFE is carried out at high pressures
which makes the process more dangerous.
* The expense of high-pressures.
And the main disadvantages are:
*
Supercritical fluids are usually gaseous at room temperature and
atmospheric Pressure. Therefore recovery of the extract is relatively straight
forward, by decreasing the pressure in a controlled manner; the extracted
material can be deposited.
* Solvent strength can be changed by
changing density.
* Higher diffusion coefficients found in
supercritical fluids give rise to higher mass transfer, and much faster
extraction.
* Because SFE is carried out at room
temperature, thermally unstable compounds can be extracted.
Chromatography
Supercritical
fluids as reaction media (top)
Supercritical fluids can be used as
reaction media, making use of their property of greater diffusivity and
tuneable density and thus solubility properties. However the reaction processes
may be different under supercritical conditions. This becomes an important
consideration particularly when organic synthesis reactions take place because
these often involve many complex stages.
Supercritical water
oxidation (top)
[Chemical Engineering Progress April 1995 pg18] Organic materials
can be oxidised with great efficiency in supercritical water. SCWO is compared
to a similar process called wet oxidation. WO has the disadvantage that it
leaves acetic acid and ammonia in the waste stream, which have to be removed by
biological means and the exhaust gases have high levels of carbon monoxide.
SCWO allows ammonia production to be reduced to about 5% and lowers carbon
monoxide levels to an acceptable level.
The main point of concern about the SCWO method is solids handling
within the system. It has been found by researchers at the University of Tulsa
that the addition of a catalyst makes the supercritical oxidation more
effective. Catalysts such as V2O5 and MnO2/CeO are being tried. NATO is developing the SCWO for use in
destroying various chemical weapons, particularly in the Iraqi chemical weapon
stockpile. This contains such items as sulphur mustard gases and nerve agents.
NATO engineers have developed mobile SCWO reactors with capacities of 400l/day.
The oxidant used is sometimes compressed air, but carries the penalty of 80%
inert Nitrogen. Liquid oxygen can be cryogenically pumped and vaporised into
the reactor and may be economical for large systems. Hydrogen peroxide is more
expensive but is relatively safe to handle.
Pipe flow reactors are the simplest but
may be more subject to plugging by salts and erosion by solids. The vertical
vessel is most convenient for salt/solid separation from the reaction medium
and may be best for high solids feeds or when neutralisation of the waste
Supercritical
fluids as reactant solvents (top)
[M. Jobling] Hydrocarbons have such a wide
variety of applications in synthetic chemistry that it seems such a waste that
a significant proportion of the constituents of crude oil, the alkanes, are
very unreactive. As a result a huge research effort has taken place in response
to the massive growth in synthetic chemistry in industry since the war. It was
found by the end of the 1960s that transition metal complexes could be made to
activate C-H bonds (alkanes have no pie-bond to react with the metal centre, so
it has been suggested that the unbroken C-H bond co-ordinates to the metal
centre, donating some electron density and thus weakening the C-H bond). One
problem however still remained in solving this problem, and that was to find an
appropriate solvent for the activation. The solvent must have reasonable
solvating power (capable of dissolving the activation complex), there can be no
C-H bonds in the solvent and the solvent must be chemically inert.
Supercritical fluids were suggested a s an appropriate solvent. Supercritical
Xenon. ScXenon has been used at Nottingham as a solvent for organometallic
photochemical reactions, so it can dissolve organometallic compounds and it
obviously does not have any C-H bonds. Supercritical fluids have the advantage
of being completely miscible with reactant gases. Conventional solvents give
rise to an additional problem in C-H activation reactions, once the reaction
has taken place, recovering the product is often accompanied with complete or
total decomposition. With supercritical
fluids the products can be removed by reducing the pressure. However the process
must be developed so that when the pressure is reduced the product does not
just coat the reaction vessel.
Supercritical fluids can be used as
cleaning solvents. Cleaning narrow tubes and other small apparatus can be very
difficult with conventional solvents. Supercritical fluids provide much better
cleaning.
Polymer Synthesis
(top)
Flow Reactors [J. Banister ORGANOMETALLIC
REACTIONS IN SUPERCRITICAL FLUIDS: Development of a Flow Reactor 1994 pg.66-80]
Flow reactors are the most efficient way
of dealing with supercritical reactions. A flow reactor is a continuous, with
feed flowing into the reactor and product removed from the reactor all the
time. The design of such reactors concentrates on keeping the volume within the
reactor to a minimum. This is because it is cheaper and easier to keep only a
small section of the apparatus at the required high pressure. Flow reactors have the following advantages:
* Solution, reaction and product removal
can occur simultaneously.
* Only a small proportion of the solution
is involved n the reaction (associat4d with keeping the volume down).
* Since it can be continuous, the overall
volume of the reactor can be less than for a batch reactor. This makes economic
sense, since a smaller volume will require less cost on high-pressure
equipment.
* Since supercritical fluids have a high
viscosity, small diameter, highly flexible steel tubing can be used, which
keeps the overall volume of the reactor to a minimum.
* Deposition of solid product can be
controlled under inert condition. By
having two valves, one at the inlet and one at the outlet, high-pressure
'stabilising' conditions can be maintained at the point of precipitation when
the product is deposited by the rapid expansion of the fluid.
DETAILS OF A FLOW
REACTOR (top)
A continuous flow reactor will have a pump
at one end, which supplies the fluid at high pressure, maintaining a small
pressure gradient across the reactor. The type of pump used may be very
different depending on whether or not the reactor is laboratory scale or
industrial scale. In a laboratory it makes sense to use a syringe pump because
a syringe can maintain a constant pressure, but it has a limited volume. In a
larger scale a compressor is an alternative, however the pressure fluctuates
with time as the valves poem and close, which must be damped out.
In working reactors a lot of consideration
is put into how the volume is to be kept as small as possible. All individual
components of a flow reactor have a low volume. Even the taps are kept so there
is no dead volume. The connection between the tubing and tap body is also
designed to keep internal volumes to a minimum. At the start of the flow reactor, the reactants must be dissolved
into the supercritical fluids. This can be done by a supercritical extraction
vessel (removes substances into supercritical fluid). The solvent can be
collected by either bubbling the effluent through another solvent or by passing
the solvent over a nn adsorbent. This provides additional problems to industry
in finding a solvent that the product can easily be removed from. At the other end there will be a valve that
will reduce the pressure to atmospheric in a controlled manner.
Polymer Impregnation [M Jobling 5.1-5.4][sc-times.com David L.
Tomasko 'sorption processes in Supercritical Fluids']
Supercritical fluids can be made to
dissolve into polymers with very interesting effects. The fluid will pass into
the polymeric matrix as a result of a high-pressure gradient supplied. Once the
pressure is removed the internal pressure will exceed the external pressure of
the atmosphere and the polymer will expand. The polymer eventually returns to
its original size. However any dissolved substances that existed within the
supercritical gas will have become deposited within the polymer. This can
become very useful if the dissolved substances have some useful properties,
such as colour. The 'wettability' or and density of a polymer can be changed by
similar impregnation. Liquid organic swelling agents do exist, but are hard to
separate after impregnation.
Industry is under constant pressure from
government organisations and pressure groups to increase their environmental
awareness and limit wastes released into the surrounding as much as possible.
Dr. Lalit Chordia of the supercritical Times says:
'As a result of the Montreal Protocol and
the Kyoto Conference, there is a sense of urgency and need to develop
industrial processes that are energy efficient and environmentally-compatible'.
' Research of supercritical fluids as
reaction medium and as a solvent medium has seen recent resurgence, driven by
needs to satisfy environmental regulations using efficient processing and
separation techniques.'
ALTERNATIVES TO
SUPERCRITICAL FLUIDS (top)
Supercritical technology is developing so
fast, with numerous lists of users for supercritical fluids. However I have
noticed that there is an understandable enthusiasm by the supercritical
researchers to apply their technique to everything even when it appears that
such technology is not required. An Article in 'The Chemical Engineer' (July 94
pg. 3) brought to my attention that alternatives to supercritical operation
exist and are more practicable in some circumstances. James Green suggests that
WAO (Wet Air Oxidation) is essentially a lower energy alternative to SCWO. WAO
uses milder process conditions (280-330C and 62-120 bar). The efficiency is not
quite as high (95% compared to 99% for SCWO). He suggests that if the milder
conditions do not produce the required purification of the water, it can be
considered with another technology such as biotreatment. He suggests that using
the SCWO process could sometimes be, like using "A Sledgehammer to Crack a
Nut!"
CONCLUSION (top)
There are clearly many new and varied
technologies available making use of supercritical fluids. The development of these
techniques is encouraged by the environmental advantages of using non-toxic
solvents for example. However the cost of such equipment is inhibiting the
technologies use at its full potential. We live in a World where individual
companies have to fit the bill for environmentally friendly technologies. The
high capital costs are sometimes weighed against the low operating costs that
exist when the critical point is near ambient. However the operating costs can
be significant if the pressures required are high and the temperatures are
equally large. Equipment costs have been traditionally high for supercritical
technologies because the equipment is adapted from other areas of chemical
processing rather than addressing specific equipment needs of supercritical
fluids. European companies are selling
the equipment as custom equipment instead of standard
designs[sc.times.com:Editorial]. A few American firms have developed equipment
specifically for supercritical work and are able to produce it much more cheaply.
I believe that as the technology become more widespread and engineers develop
experience and confidence in its use and design it will become a technology
that will influence everything from the dry cleaning of our clothes to the
production of the polymers in our drink bottles.
Appendix 2 (top)
Other
Uses of Supercritical Fluids
Activation of the C-H bond in
supercritical solution.
Hydrocarbons have such a wide
variety of applications in synthetic chemistry that it seems such a waste that
a significant proportion of the constituents of crude oil, the alkanes, are
very unreactive. [M. Jobling20 ]. As a result a huge research effort has taken
place in response to the massive growth in synthetic chemistry in industry
since the war. It was found by the end of the 1960s that transition metal
complexes could be made to activate C-H bonds (alkanes have no p-bond to react
with the metal centre, so it has been suggested that the unbroken C-H bond
co-ordinates to the metal centre, donating some electron density and thus
weakening the C-H bond). One problem however still remained in solving this
problem, and that was to find an appropriate solvent for the activation. The
solvent must have reasonable solvating power (capable of dissolving the
activation complex), there can be no C-H bonds in the solvent and the solvent
must be chemically inert. Supercritical fluids were suggested as an appropriate
solvent. Supercritical Xenon has been used at Nottingham as a solvent for
organometallic photochemical reactions, so it can dissolve organometallic
compounds and it obviously does not have any C-H bonds. Supercritical fluids
have the advantage of being completely miscible with reactant gases.
Conventional solvents give rise to an additional problem in C-H activation
reactions, once the reaction has taken place, recovering the product is often
accompanied with complete or total decomposition. With supercritical fluids the products can be removed by reducing
the pressure. However the process must be developed so that when the pressure
is reduced the product does not just coat the reaction vessel.
Polymer Impregnation
Supercritical fluids can be made
to dissolve into polymers with very interesting effects (M Jobling) 20. The fluid
will pass into the polymeric matrix as a result of a high-pressure gradient
supplied. Once the pressure is removed the internal pressure will exceed the
external pressure of the atmosphere and the polymer will expand. The polymer
eventually returns to its original size. However any dissolved substances that
existed within the supercritical gas will have become deposited within the
polymer. This can become very useful if the dissolved substances have some
useful properties, such as colour. The 'wettability' or and density of a
polymer can be changed by similar impregnation. Liquid organic swelling agents
do exist, but are hard to separate after impregnation.
Liquefaction of Coal
Amestica. L.A. et al1 describes
the use of supercritical water/CO/solvent media in the catalytic liquefaction of
coal. CoMo and Tetralin sulphided catalysts have been used, and it was noted
that the supercritical conditions enhance the solubility and diffusivity of the
solvent, and minimize mass transfer effects by operating in a single-phase
system. A similar process is described by Townsend et al27, where they note
that the observed extract yields correlate well with the solvent critical temp.
Both the importance of thermal fragmentation of the coal during extraction at
high tempts and the significance of the supercritical region on solubility were
included in the article.
Supercritical fluids can be used
as cleaning solvents. Cleaning narrow tubes and other small apparatus can be
very difficult with conventional solvents. Supercritical fluids provide much
better cleaning. The synthesis reactions also include polymer Synthesis among
the vast other varieties, only a few of which I have mentioned.
12. References
Key:
· The date of publication is in brackets.
· The volume number is underlined
· Where possible I have included the publisherand the place
of publication.
1. [Amestica L.A. and Wolf E.E. Catalytic Liquefaction of coal with supercritical
water/CO/solvent media Fuel (1986) 65,
pp.1226 University of Notre Dame, IN USA, Butterworth & Co.]
2. [Banister J.A, Ph.DOrganometallic Reactions In Supercritical
Fluids: Development of a Flow Reactor, pp. 7]
3. [Barton P. Supercritical separation in aqueous coal
liquefaction with impregnated catalyst, Ind.Eng.Chem.Process Des. Dev (1983)
22 pp.589 The Pennsylvania State
University, American Chemical Society ]
4. [Brennecke. J.F, Chemistry and Industry New applications of
supercritical fluids Chemistry and Industry
(1996) pp.831 University of
Notre Dame, IN, USA ]
5. [Brock E.E., Savage PE and Barker J.R., A reduced mechanism
for methanol oxidation in supercrit Chem.Eng.Sci, 53 No.5 pp 857 (1998)
University of Michigan, Peragmon Publishers Ltd.]
6. [Caignard de la Tour, C Ann. Chim.(1822), 22 pp.410]
7. [Caruana.C.M. Supercritical water oxidation aims for
wastewater cleanup Chemical Engineering Progress April 1995 pp. 10 New York, NY
10017 American Institute of Chemical Engineers (AIChE)]
8. [Caruana C.M. Technologies breaking new ground for soil
remediation, Chemical Engineering Progress October 1995 pp.23. New York NY
10017 American Institute of Chemical Engineers (AIChE)]
9. [Chen Shaw-Horng Rough-Hard-Sphere theory for diffusion in
supercritical carbon dioxide, Chemical Engineering Science Supercritical Fluid
Extraction, (1983) 38 pp.655 University of Rochester, NY, USA, Pergamon Press
Ltd. ]
10. [Deshpand GV, Holder GD, Bishop AA, Gopal J and Wender I,
Fuel Extraction of coal using supercritical water (1984) 63 , University of Pittsburgh, PA, USA,
Butterworth & Co.]
11. [Diepen G.A.M.; Scheffer, F. E. C. J. Am. Chem. Soc., 1948,
70, 4084.]
12. [Edward W. Cook,
Oil-shale technology in the USA Fuel July 1974, 53, pp146 Rocky Flats Reasearch center,
Colorado].
13. [George C. Kennedy, A portion of the system silica-water
Econ. Geo. (1950) 45 pp 629 Division of Geological Sciences, Harvard
University].
14. [Green J, author of a letter in 'The Chemical Engineer'
(1994) pp. 3. 122 Kiln Road, Benfleet, Essex]
15. [Hannay J.B. and Hogarth J., Proc. Roy. Soc. (London) ser. A
(1879) 29 pp.324]
16. [Harmon T.C. and Jackson D.Y, Dispersion and diffusion in
porous media under supercritical conditions, Chem.Eng.Sci., (1999) 54 pp.357,
University of California, Pergamon Press Ltd.]
17. [Houser T.J., Tiffany D.M., Li Z, McCarville M E. and
Houghton M.E. Fuel Reactivity of some organic compounds with supercritical
water (1986) 65 pp827 Western Michigan
University, Butterworth & Co.]
18. [Humphrey JL. Separation Processes playing a critical role,
Chemical Engineering Progress (1995)
pp..36. New York 10017 American Institute of Chemical Engineers (AIChE)]
19. [Ingerson E., Relations of critical and supercritical
phenomena of solutions to geologic processes, Econ. Geol. (1934) 29 pp. 454,
Yale University, New Haven, CONN.]
20. [Jobling M, Ph.D Organometallic Photochemical Reactions in
Supercritical Fluids (1992) Chapters 1-5].
21. [Knopf A. U.S. Geol. Surv. (1918) Prof. Paper 114 pp44 ]
22. [McHugh, M.; Krukonis, V. 'Supercritical Fluid Extraction,
Principles and Practice'., 1986 Boston, USA,
Butterworths]
23. [ Mackay. M. E. and
Michael E. Paulaitis Industrial and Engineering Chemistry Solid Solubilities of
Heavy Hydrocarbons in Supercritical Solvents (1979) 18 No.2 pp149 American Chemical Society]
24. [McHugh, M.A.; Paulaitis, M. E J. Solid solubilities of
naphthalene and biphenyl in supercritical carbon dioxide, Chem.Eng.Data,
(1980), 25, 326, University of Delaware, Newark, American Chem.Soc.]
25. [McLaughlin H.S. Current solvent regeneration applications,
Chemical Engineering Progress (1995) -
pp. 47 American Institute of Chemical Engineers (AIChE) NY 10017]
26. [Santesson, J. Report of the Nato Advanced research into the
desturction of chemical weapons. Pg1-30].
27. [Sundaresh Ramayya, Andrew Brittain, Carlos DeAlmeida,
William Mok and Michael Jerry Antal, Jr Fuel Acid-catalysed dehydration of
alcohols in supercritical water, (1987)
66 pp. 1365 University of
Hawaii, Butterworth & Co.].
28. [Townsend S.H. and .Klein M T Fuel Dibenzyl ether as a probe
into the supercritical fluid solvent extraction of volatiles from coal with
water. 1985 64 pp635 Butterworth &
Co. University of Delaware]
29. [Wandler R. and Baiker A. CatTech. The magazine of catalysis
sciences, Technology and innovation (2000).7. ]
30. [Williams D.F., Chem.Eng.Sci. Extraction with supercritical
gases (1998) 36. pp. 1769 Coal Research establishment, Gloucestershire,
Pergamon Press Ltd].
31. [Zhuze T.P., Vesnik Akad. NaukS.S.S.R (1959) 29 47]
Internet References
32. [www.nottingham.ac.uk/supercritical. Graph 1. Page 1]
33. [www.nottingham.ac.uk/supercritical/scwater.html]
34. [www.sctimes.com. editorial, Dr.Lalit Chordia, 1985, Thar
Designs, Carnegie-Mellon University].
35. [www.kobelco.co.jp/p108/p14/sfe04.htm]