Fractures of Adult metaCarpal shafTS (FACTS) Study
Study title
Surgical and Non-Surgical Treatment for Metacarpal Shaft Fractures in Adults: an Observational Feasibility Study
Overview
The objective of the FACTS study is to investigate the feasibility and acceptability of conducting a randomised controlled trial (RCT) to identify the most effective way of treating metacarpal shaft fractures in adults. FACTS is a dual centre observational feasibility study, consisting of a prospective cohort study, qualitative interviews and focus groups and a nested study assessing the use of text message in research studies.
The study is led by Clinical Research Fellow Rowa Taha and Clinical Associate Professor Alexia Karantana of Nottingham University Hospitals NHS Trust. Patients will be recruited from Queen’s Medical Centre, Nottingham University Hospitals NHS Trust and the Pulvertaft Hand Centre, Royal Derby Hospital, University Hospitals of Derby and Burton NHS Foundation Trust.
The study is funded by a National Institute for Health Research (NIHR) Doctoral Research Fellowship awarded to Miss Rowa Taha.
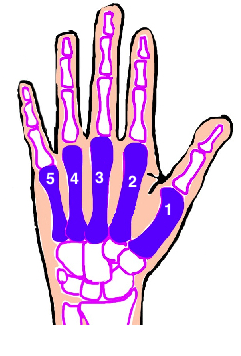
Image from www.bonetalks.com
Key facts
This study is currently recruiting!
Sponsor: University of Nottingham
Sponsor reference: 20018
Ethics reference: 20/EE/0124
IRAS project ID: 279115
ISRCTN registration: 13922779
FACTS study protocol 
Contact details
Miss Rowa Taha
Centre for Evidence Based Hand Surgery
Room WC1373, C Floor, West Block
Queen’s Medical Centre
Nottingham, NG7 2UH
Tel: 07973793844
Email: FACTS-study@nottingham.ac.uk
Information for patients
What is the FACTS study about?
Metacarpal shaft fractures are breaks (also known as fractures) in the middle part of the bones in the hand. They are very common injuries in young, working-age people. At the moment, we do not know how best to treat them, so patients get different care in different parts of the country. The best way of finding out is to do a trial comparing treatments.
This study will assess whether a future trial is possible and how best to carry it out, to tell us the most effective way of treating these injuries.To find out more download our patient information leaflets:
FACTS study patient information leaflet 
FACTS qualitative study patient information leaflet 
We are inviting patients who have had a break in the middle part of the index to little finger metacarpal. You must be over 16 years old to take part in this study.
We are recruiting patients from hand fracture clinics at Queen’s Medical Centre, Nottingham and the Pulvertaft Hand Centre, Derby. If you are eligible, a research associate will discuss the study with you during your clinic visit.
You will be asked to attend one clinic visit in addition to your routine clinical care and to complete some questionnaires remotely. If you are willing to talk to us about your injury and taking part in research, we may invite you for an interview and/or focus group.
The questionnaires are short and easy to complete and all reasonable expenses, such as travel and parking costs associated with attending the research clinic, patient interviews and focus groups will be fully reimbursed.
Frequently asked questions
Q. Will my travel expenses be covered?
Yes. We will cover all your travel/parking costs for any visits you make as part of the study.
Q. Will I be paid to take part in this study?
No. You will not be paid an allowance for taking part in this study but vouchers will be offered if you take part in the interviews or focus groups linked to the study.
Q. What are the possible benefits of taking part?
The information we get from this study will help us to improve treatment for other people like you in the future.
Q. What are the possible disadvantages and risks of taking part?
Taking part in this study does not affect the usual care you receive. We will just be monitoring how you and your hand are recovering, so there are no risks to taking part in this study.
To find out more download our leaflets or contact us.
FACTS study patient information leaflet 
FACTS qualitative study patient information leaflet 