 Reactions
in Supercritical water
Reactions
in Supercritical water
Introduciton
Water has obvious attractions as a solvent for clean chemistry. Both near-critical
and supercritical water (scH2O) have increased acidity, reduced
density and lower polarity, greatly extending the possible range of chemistry
which could be carried out in water. scH2O has already been
studied extensively as a medium for the complete destruction of hazardous
and toxic wastes.
In this Project, our aim is to establish how scH2O can be
exploited most effectively for organic synthesis leading to useful products.
We have three objectives:
-
Developing chemistry in near-critical and supercritical H2O
with particular emphasis on partial oxidation processes and on organic
synthesis of relevance to the fine chemicals industry.
-
Monitoring and understanding corrosion in scH2O under these
conditions.
-
Developing high pressure techniques, appropriate for studies of chemistry
in scH2O on both laboratory and technical scale.
We have already devised prototype apparatus, a miniature batch reactor
and a larger scale flow reactor both of which can handle water at any temperature
from ambient to supercritical, without many of the experimental difficulties
of handling scH2O which have previously discouraged most chemists
from entering this area.
Background
As water is heated towards its critical point (Tc 374 oC,
Pc 218 atm.), it undergoes a transformation considerably more
dramatic than that of most other substances. It changes from the familiar
polar liquid to an almost non-polar fluid. The change occurs over
a relatively wide temperature range; even at 200 oC, the density
has dropped to 0.8 g/ml and, at Tc, the fluid becomes miscible
both with organics and with gases. Diffusivity increases and the acidity
is enhanced more than would be expected purely on the basis of higher temperatures.
Over the past decade, a major research effort has been focused on the total
oxidation of toxic organics in scH2O, "incineration
without a smokestack". The process is highly effective but there can
be serious problems of corrosion associated with
large scale
waste destruction, so serious indeed that many chemists have been discouraged
from even contemplating possible uses of scH2O as a medium for
chemical reactions. Recently, however, there have been a number of reports
which show that high temperature and supercritical water can be used constructively
for reaction chemistry. It is clear that increasing the temperature renders
water increasingly acidic and favours ionic processes over radical or purely
thermal pathways. The challenge lies in finding how to exploit these effects
safely on a larger scale over a wide range of chemistry.
Our Project
Our Project is centred on the use a flow system where safety problems are
inherently less than in a sealed batch reactor. In collaboration with a
specialist equipment company NWA GmbH, we have constructed a simple prototype
flow apparatus at Nottingham for handling water up to 450 °C. We already
have significant experience in the development of miniature
flow reactors. A schematic of the supercritical water flow reactor
used in Nottingham is shown below.
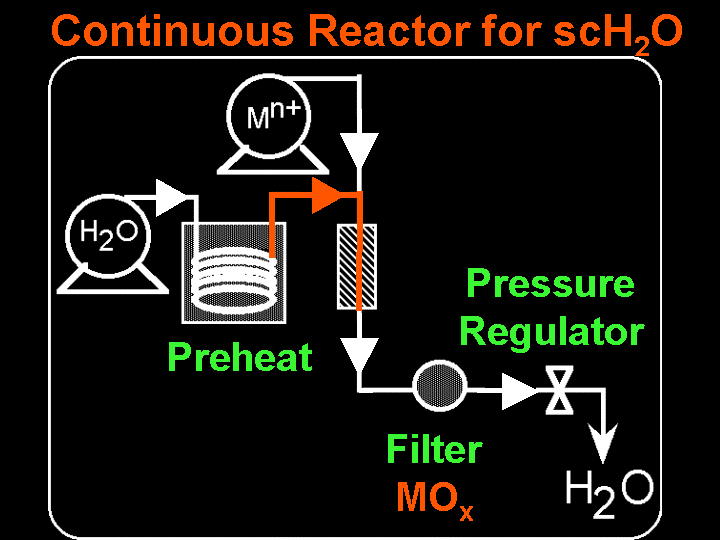
Specific goals include:
-
The development of a miniature batch reactor to allow reactions to be tested
on a small scale and of a flow reactor which has the twin advantages of
inherent safety and of straightforward scale up, at least to technical
scale, where there are possible commercial applications.
-
An investigation of partial oxidation processes in near-critical and scH2O,
with the aim of establishing the extent to which the process can be controlled
and of identifying the most effective oxidants
-
The screening of a wide range of reactions of potential value in organic
synthesis to identify those which can be carried out selectively and efficiently
in near-critical and scH2O.
Further Information
For further information please contact M.
Poliakoff.
Recent Publications from Nottingham
-
Hydrolysis and Saponification of Methyl Benzoates - A green procedure in
high temperature water, Aleman, P.A.; Boix, C.; Poliakoff, M., Green Chemistry,
April 1999, 65.
-
New Directions in Inorganic and Metal-Organic Coordination Chemistry in
Supercritical Fluids, Jawwad A. Darr and Martyn Poliakoff, Chem. Rev.1999,
99,
495-541.
-
Efficient H-D Exchange of Aromatic Compounds in Near-Critical D2O
Catalysed by a Polymer-Supported Sulphonic Acid, C. Boix and M. Poliakoff,
Tetrahedron
Letters, 1999, 40, 4433-36.
-
Reduction of Aromatic Nitro-Compounds Using Metallic Zinc in High Temperature
Water, C. Boix and M. Poliakoff, J. Chem. Soc. Perkin Transactions 1,
1999, 1487-90.
-
A Continuous and Clean One-Step Synthesis of Nano-particulate Ce1-xZrxO2
Solid Solutions in Near-Critical Water. A. Cabañas, J. A. Darr,
E. Lester and M. Poliakoff. Chem. Commun. 2000, 901-2.
For good surveys of scH2O, see
-
Shaw, R. W.; Brill, T. B.; Clifford, A. A.; Eckert, C. A.; Franck, E. U.
Chem.
Eng. News, 1991, 69, 26;
-
Savage, P. E.; Gopaian, S.; Mizan, T. I.; Martino, C. J.; Brock, E. E.
A.
Inst. Chem. Eng. J., 1995, 41, 1725.
-
Organic chemical reactions in supercritical water. Savage P. E., Chemical
Reviews, 1999, 99: 603-621
University of Nottingham Welcome
page
Inorganic
Chemistry Welcome page
The Clean Technology
Research Group Welcome page
Page created by: Simon Poliakoff
Created: July 1997
Last Revised: January 2001
 Reactions
in Supercritical water
Reactions
in Supercritical water
 Reactions
in Supercritical water
Reactions
in Supercritical water
