
The HORIBA LabRAM HR Confocal Raman Microscope available at the nmRC
Confocal Raman Microscopy
Raman spectroscopy is a non-invasive optical spectroscopy technique used to determine the chemical identity and state of a sample by its unique vibrational modes. The inelastic scattering of light from the sample is measured, with shifts in the energy of the scattered photons being representative of the sample chemistry.
The Raman microscopes at the nmRC can be used as traditional Raman spectrometers to obtain vibrational spectra, but the most powerful feature of these instruments are their ability to collect information from any array of points on - or in - the sample to rapidly produce false colour images (1D, 2D or 3D), where the brightness, contrast and colour of the image is used to convey the science of the sample.
The confocal set-up of the microscopes gives rise to clearer images (by reducing contributions from out-of-focus background regions) and confers the ability to perform depth-profiling studies. These instruments are ideally suited for materials and pharmaceutical characterisation, tissue imaging and chemical identification.
Get in touch with us to discuss your Raman requirements.
Key Features
- Conventional and inverted microscope geometries.
- Multiple excitation wavelengths available.
- Multiple gratings available for standard or high spectral resolution spectroscopy.
- Controllable confocality for standard or high spatial resolution hyperspectral imaging.
- SWIFT™ imaging capability for ultra-fast mapping applications.
- EasyNav™ software module for optimal Raman imaging.
- Multivariate analysis (MVA) software module for advanced data processing.
- KnowItAll™ database for spectral searching, analysis and data mining.
- Additional software modules tailored for specific applications.
- Linkam THMS600 variable temperature stage (-196 to 600 oC).
- Custom-designed flow cell for correlative 'in-situ' dissolution and elution studies.
- Bespoke cell for Raman spectroelectrochemistry studies.
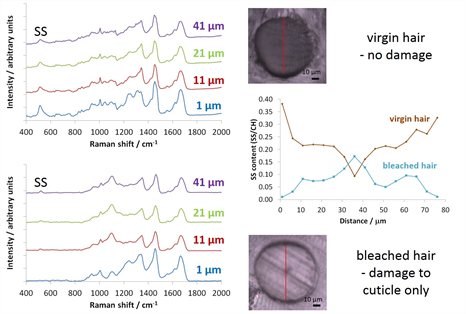
One-dimensional mapping: chemical damage to hair
Hair is susceptible to changes and damage induced by a number of factors, including the application of bleaching and colouring treatments. Using confocal Raman microscopy it is possible to spatially locate differences in the composition of (blonde) hair and thus evaluate the extent of damage induced by such chemical treatments. In this study, we line-mapped cross-sections of treated and untreated hair and using the intensity of diagnostic band at 540 cm-1 associated with S-S cross-linkages determined that whilst bleaching does destabilise the structure of the hair at the surface (cuticle), it does not penetrate significantly into the centre (cortex).
Two-dimensional mapping: mechanical exfoliation of graphite
Graphene has been hailed as the wonder material of the twenty-first century. It can be readily procured by simple exfoliation of bulk graphite using sticky tape with the properties of the resultant material dependant on the number of graphitic layers present. Using confocal Raman microscopy it is possible to spatially locate the presence of mono-, bi-, tri- and tetra-layer graphene using the position and symmetry of the diagnostic band at ~2600 cm-1 (2D band). In this study, we used two-dimensional mapping to probe the layering in this few-layer graphene sample and found it to comprise a mixture of layers, a fact that would have been indeterminable from the optical image alone.