Products
MAbs are often considered high risk as either not effective or too toxic but these have been de-risked by, employing new platform technologies developed within the Centre and, conducting extensive screening assays to generate the ideal therapeutics.
A number of mAbs have been produced and are at various stages of development:
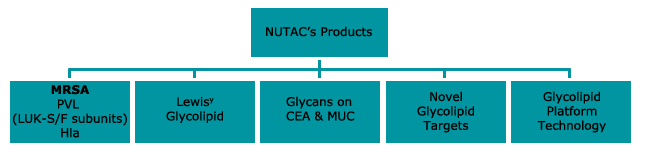
Some of these new mAbs are available for licensing whilst others continue to be developed in-house.