Bernard Soulier Syndrome - molecular details
Mutation of the GPIb-V-IX complex result in abnormal appearance and functioning of platelets resulting in Bernard Soulier Syndrome (BSS), a condition first described by Bernard J and Soulier J.P (Semin Hop Paris (1948) 24:3217-3223). It is a rare hereditary bleeding disorder most commonly with an autosomal recessive inheritance and diagnosed based on prolonged skin-bleeding time, very large platelets in reduced number (macrothrombocytopenia) and defective ristocetin-induced platelet agglutination (Pham and Wang, 2007).
Bernard Soulier Syndrome is characterized by little or no expression of GPIb-IX on the surface of platelets which in turn has the same effect on GPV. There have been a number of mutations associated with BSS patients that have been mapped to the GPIbα, GPIbβ and GPIX subunits demonstrating that all three subunits are required for effective surface expression of the complex on platelets (McEwan et al., 2011).
Mutations that occur can be separated into three separate categories including missense mutations, nonsense mutations and frameshift insertions or deletions (Lanza, 2006). Nonsense or frameshift mutations in the GPIb-IX genes give rise to the production of significantly shortened or changed subunits that lacks large portions important for the structural integrity of the subunit and/or the assembly of the entire receptor complex. The extracellular and transmembrane domains are typically involved in the assembly of the GPIb-IX complex and BSS mutations tend to be restricted to these domains as opposed to occurring in the cytoplasmic domain (Li and Emsley, 2013).
Missense mutations in BSS can disturb GPIb-IX assembly. This can occur by disrupting the folding of the host domain or considerably destabilizing it. For example, mutations that modify specific cysteine residues may interfere with disulfide bond formation. A missense mutation causing BSS can also interrupt interaction of the GPIb-IX subunits preventing effective assembly of the receptor complex (Li and Emsley, 2013). Below are details of each mutation mapped on the amino acid sequence of the subunits (sequence files generated by Mathieu Fiore, Hôpital Xavier Arnozan, Pessac, France)
1. GPIbα BSS mutations (pdf)
2. GPIbβ BSS mutations (pdf)
3. GPIX BSS mutations (pdf)
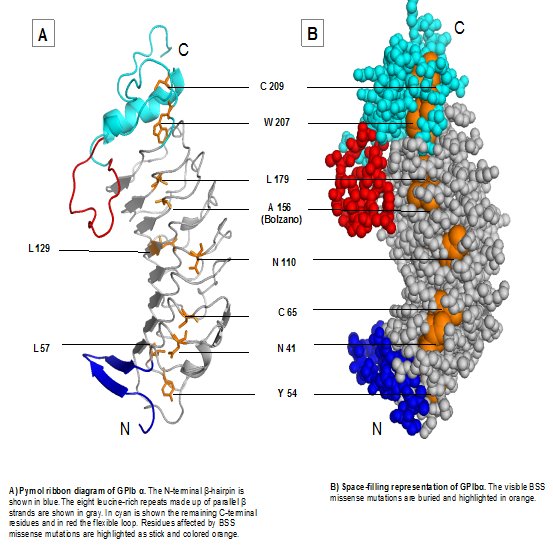
Ribbon and space-filling representations of GPIbα indicating potential residues affected in Bernard Soulier Syndrome (McEwan et al., 2011) (above)
Similarly, the GPIbβ ectodomain, which is a membrane proximal region of the receptor can also be affected by mutations associated with BSS
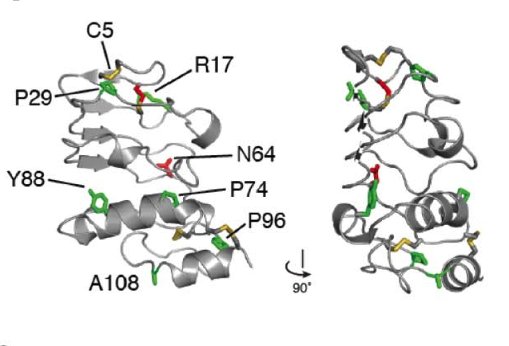
Ribbon representation of GPIbβ indicating potential missense mutations associated with BSS (McEwan et al., 2011) (above)