Biomaterial surface design
As the first point of contact for cells, the surfaces of biomaterials play an important role in the biological response of tissue to a biomedical device. By modifying the surface of a material, this response can be studied and controlled.
We are focusing on the use of biologically based molecules such as peptides and proteins to alter the surface properties of a material. Activities include the development of new surfaces and surface fabrication tools and the build-up of a detailed understanding of how these surfaces affect cell-material interactions.
We explore various ways to fabricate peptide-based surfaces including the grafting of peptides directly from the surface either in a solid phase peptide synthesis-like manner or via N-carboxy anhydride (NCA) polymerisation and the coating of surfaces with peptide-containing polymer films
Solid phase peptide synthesis from surfaces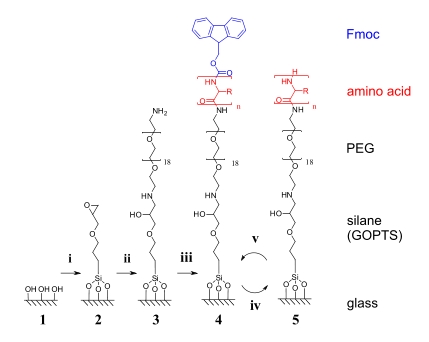
Construction of peptide surfaces with controlled amino acid sequences directly on the surface using a solid phase peptide synthesis-like approach. Glass surfaces are functionalised with epoxy groups to attach a short PEG-diamine spacer which provides a flexible, amine functionalised surface from where solid phase peptide synthesis is carried out directly on the glass surface. Peptide sequences are thus built up stepwise from the solid substrate using Fmoc-protected amino acids.
Polypetide materials and surfaces via NCA polymerisation
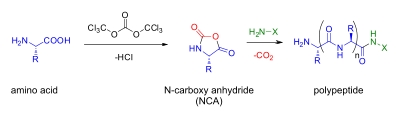
NCA polymerisation used to synthesise polypeptides. Side chain protected amino acids are converted to a reactive N-carboxy anhydride (NCA). Ring opening polymerisation of the NCA amino acids can be initiated with amines, yielding a polypeptide with well controlled molecular weight and polydispersity and only CO2 as side product. Random copolymerisations of more than one amino acid can be performed.
Copolypeptide design
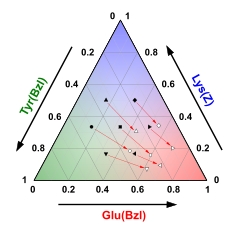
Ternary diagram exploring the composition in a peptide based terpolymer. Three side chain protected amino acid NCAs based on glutamic acid, lysine and tyrosine were copolymerised. Study of the reaction kinetics and copolymerisation parameters provides insight into the reactivity of the monomers and the composition of the copolymers, demonstrating that the initial composition of the copolymer deviates significantly from the feed mixture, incorporating more glutamic acid NCA.
Ongoing projects:
- Development of surfaces with well-defined peptide concentrations for the development and advancement of peptide surface analysis. (in collaboration with Alex Shard and Morgan Alexander)
- Peptide surfaces that control neuronal stem cell differentiation. (in collaboration with Noah Russell)
- Grafting of polypeptides with specific secondary structures from the surface.
References:
-
Canning, A.; Pasquazi, A.; Fijten, M.; Rajput, S.; Buttery, L.; Aylott, J. W.; Zelzer, M., Tuning the conformation of synthetic co-polypeptides of serine and glutamic acid through control over polymer composition. J. Pol. Sci. A: Pol. Chem. 2016, 54 (15), 2331-2336.
- Zelzer, M.; Scurr, D. J.; Alexander, M. R.; Ulijn, R. V., Development and Validation of a Fluorescence Method to Follow the Build-up of Short Peptide Sequences on Solid 2D Surfaces. Acs Applied Materials & Interfaces 2012, 4 (1), 53-58.
- Zelzer, M.; McNamara, L. E.; Scurr, D. J.; Alexander, M. R.; Dalby, M. J.; Ulijn, R. V., Phosphatase responsive peptide surfaces. Journal of Materials Chemistry 2012, 22 (24), 12229-12237.
- Zelzer, M.; Heise, A., Determination of copolymerisation characteristics in the N-carboxy anhydride polymerisation of two amino acids. Polymer Chemistry 2013, 4 (13), 3896-3904.
- Zelzer, M.; Heise, A., Terpolymerization kinetics of amino acid N-carboxy anhydrides. Journal of Polymer Science Part A: Polymer Chemistry 2014, 52 (9), 1228-1236.