Cell signalling imaging
The CSI facility is based on C Floor of the Medical School and is part of the School of Life Science Imaging (SLIM) core facility.
It provides cutting-edge imaging facilities to researchers across the University, as well as external collaborators with particular expertise in pharmacology and live cell imaging.
CSI encompasses 3 units; Fluorescence imaging, High Content Imaging and High throughput Screening
SLIM 
Resources
CSI provides access to state of the art microscopy comprising:
- Four Zeiss confocal systems: LSM880 , LSM710 META, LSM510uv META combi, LSM EXCITER. One Multi Photon system: Zeiss NLO META Confocor 3. The multi photon is also equipped with a Becker & Hickl FLIM system for lifetime imaging.
- One Zeiss widefield deconvolution-TIRF system alongside myography, fluorescence and edge detection microscopes.
The LSM880, LSM510 and Multi Photon systems all have capability for Fluorescence Correlation Spectroscopy (FCS). The LSM880, 710, 510 and Multi photon are all equipped for spectral separation experiments.
The 510uv META, TIRF and Deconvolution systems are housed within Cell Signalling culturing facility. Most of the microscopes are set up for live cell imaging with environmental control, heated stages and perfusion systems (for kinetic and long term experiments).
A wide range of live cell imaging techniques are performed in CSI, for example, GFP-tagged receptor studies, GFP complementation, kinetic studies of receptor allosterism, bacterial biofilms, cardiomyocyte stem cells, 3D and 4D timed series imaging, developmental studies, FRET, FRAP, FLIP and second messenger signalling. Laser lines available are UV, 405, 458, 488, 514, 543, 561, 594, 633nm and multi photon pulsed 720-980nm.
The confocal imaging facilities connect efficiently with the High Content Imaging and High Throughput facilities. A wide range of fluorescence microplate readers are available with the capability to perform a variety of screens. More details are available in the High Content Screening pages.
All systems within CSI are bookable on-line through the SLIM booking system and full training and consultation is provided by Tim Self and Seema Rajani.
Contact
For advice, discussions regarding experimental design &/or grant applications and access please contact Tim Self.
For training and image analysis contact Seema Rajani.
International Training Programmes
We are involved a number of International training programmes with colleagues in Brazil, Australia and Europe.
Find out more about our involvement in various training programmes.
Publications
Recent Publications:
Ivanov DP, Parker TL, Walker DA, Alexander C, Ashford MB, Gellert PR, Garnett MC. In vitro co-culture model of medulloblastoma and human neural stem cells for drug delivery assessment. J Biotechnol. 2015 Jul 10;205:3-13.
Gherbi K, Briddon SJ, Hill SJ. Detection of the secondary, low-affinity β1-adrenoceptor site in living cells using the fluorescent CGP 12177 derivative BODIPY-TMR-CGP. Br J Pharmacol. 2014 Dec;171(23):5431-45.
Mahdavi J, Pirinccioglu N, Oldfield NJ, Carlsohn E, Stoof J, Aslam A, Self T, Cawthraw SA, Petrovska L, Colborne N, Sihlbom C, Borén T, Wooldridge KG, Ala'Aldeen DA. A novel O-linked glycan modulates Campylobacter jejuni major outer membrane protein-mediated adhesion to human histo-blood group antigens and chicken colonization. Open Biol. 2014 Jan 22;4:130202.
During the last REF period, CSI resources contributed to more than 50 publications, a full list is available.
Science & Art
This video is a result of an innovative collaboration between scientists, Tim Self and Nick Holliday, in the former School of Biomedical Sciences and the artist Jo Berry. Part of a successful Wellcome Trust, Arts Council and Lottery funded grant to further public understanding of science.
The aim of the project was to use fluorescent imaging to measure drug-receptor interactions and cell signalling of the ghrelin receptor and to represent this in digital designs and 3D light boxes. The project was part of a Wellcome Trust, Arts Council and Lottery funded grant to further public understanding of science.
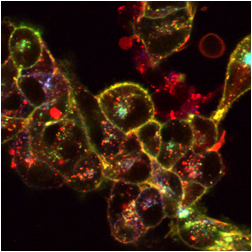
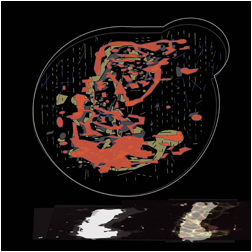
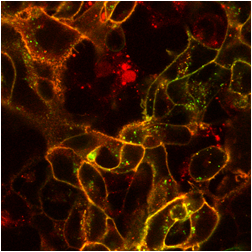
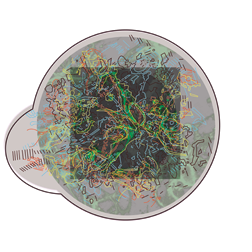
The images below are digital representations of the confocal images produced from the live cell experiments conducted during the project. Some of the original micrographs are presented alongside the artist’s images.